
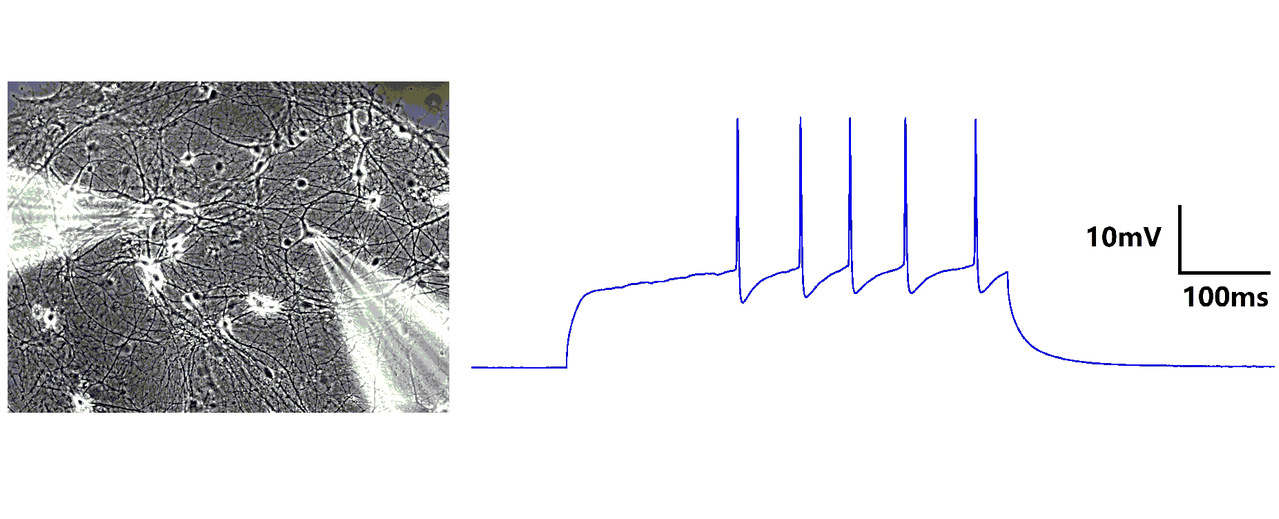
Patch clamp
Synaptic plasticity can be reflected by the changes of synaptic structures, as well as changes of postsynaptic current. The patch clamp technique is a commonly used technique in electrophysiology for studying electrical activities of neurons.
The patch-clamp technique involves a glass micropipette forming a tight gigaohm (GΩ) seal with the cell membrane. The micropipette contains a wire bathed in an electrolytic solution to conduct ions. The whole-cell technique involves rupturing a patch of membrane with mild suction to provide low-resistance electrical access, allowing control of transmembrane voltage. Perforated patch clamp is a bit different from whole-cell technique. The electrode solution contains small amounts of antifungal for antibiotic agent, such as amphothericin-B, which diffuses into the membrane patch and forms small pores in the membrane, providing electrical access to the cell interior.
Perforated patch clamp maintains endogenous levels of divalent ions such as Ca2+ and signaling molecules such as cAMP. Consequently, one can have recordings of the entire cell, as in whole-cell patch clamping, while retaining most intracellular signaling mechanisms, as in cell-attached recordings. As a result, there is reduced current rundown, and stable perforated patch recordings can last longer than one hour.
Multi-electrode array
A multi-electrode array is a grid of tightly spaced microscopic electrodes embedded in the bottom of each well in a multi-well MEA plate. Neurons can be cultured over the electrodes to create a cohesive network. The functional behavior or electrical activity of this network can be recorded. The electrodes of MEA are not only for recording, but can also be used for stimulation to continuously activate neurons. Because the base of MEA is transparent, we can observe the morphology and structure of the neuronal network at the same time. Neurons cultured on MEA can grow for weeks or seven months.