Electron microscopy and correlative microscopy
Electron microscopy, due to its extremely high resolution, has always been the main technique used for the analysis of tissue and cellular ultrastructure, molecular localization and distribution. In particular, the latest advances in cryo-EM, including cryo-EM tomography for 3D reconstruction and single-particle reconstruction, enabled us to resolve the ultrastructural details of subcellular and even protein macromolecular complex ultrastructure at nanometer and even atomic level resolution. At the same time, the evolving field of correlative microscopy combines optical and electron microscopy to achieve multi-scale correlated imaging of the same sample. By integrating the high resolution, high sensitivity, and high specificity, this technology enables us to observe and analyze the ultrafine structures such as protein complexes in subcellular systems including synapses with un precedented vision, leading to a new understanding of various biological cell structures and functions.
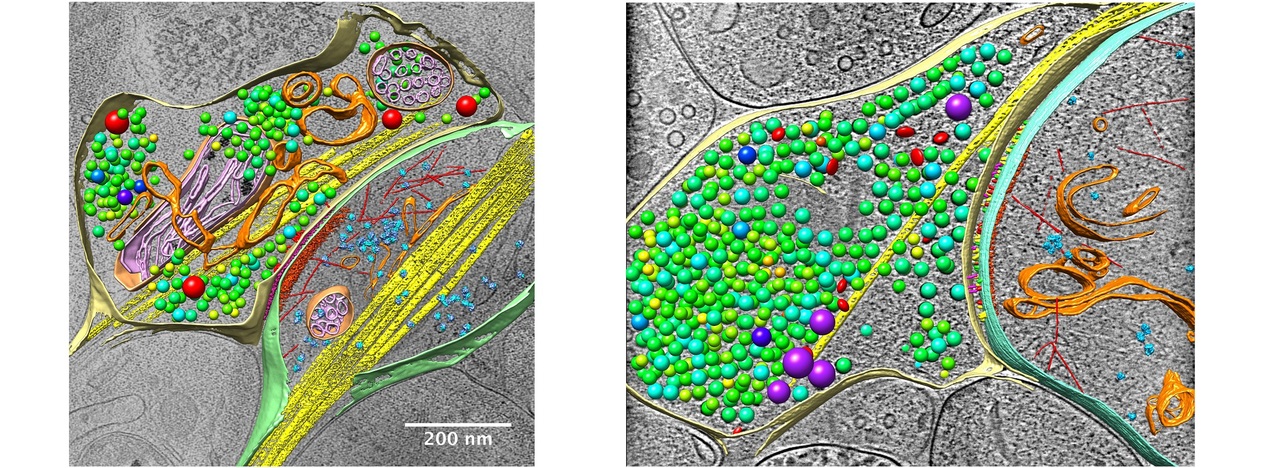
cryo-electron tomography, cryo-ET
We aim to resolve the 3D structures of various amorphous biological samples, including tissues, cells, subcellular components, and biomolecular complexes, under near-physiological conditions at nanometer resolution. The basic principle of CryoET involves rapidly freezing the sample to preserve its structures and placing it into CryoEM. By tilting the sample and collecting a series of 2D projection images, the 3D structure is reconstructed via the back-projection method. With the improvement of sample preparation technology and EM imaging quality, the quality of CryoET data has been greatly improved, enabling the in situ identification of macromolecular complexes. Combined with image processing techniques such as pattern recognition, the localization and distribution of protein molecules in specific samples, such as individual synapses, can be identified, so as to achieve quantitative analysis of the protein quantity and localization. Furthermore, by combining sub-tomogram averaging technology, it is possible to achieve high-resolution 3D structural analysis of biomolecules in situ with resolution approaching atomic level. With the development of technology, CryoET is becoming a primary research tool for the quantitative analysis of protein organization, localization and distribution at the cellular and subcellular levels, as well as in situ structural analysis of biological macromolecules.
Cryo-correlative light and electron microscopy, cryo-CLEM
Cryo-correlative light and electron microscopy (Cryo-CLEM) is materializing as a widespread approach amalgamating the advantages of both fluorescence light microscopy (FLM) as well as three dimensional (3D) cryo-electron tomography (cryo-ET) to reveal the ultrastructure of significant target molecules with specific cellular functions. Cryo-CLEM allows imaging of cells by means of fluorescence microscopy exhibiting the location of the destined molecule at high temporal and spatial resolution while cryo-ET is employed to analyze the 3D structure at a molecular resolution in close-to-physiological condition.
